 |
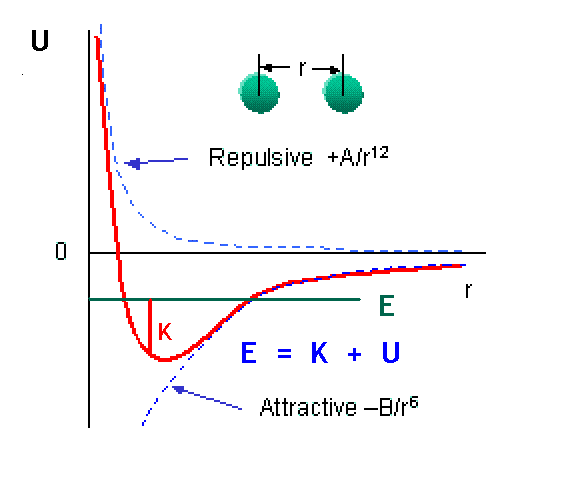
You can get an incredible amount of information from looking at the graph of a potential energy, U(r). This particular plot shows the typical potential energy between two atoms. It is weakly attractive at large distances and strongly repulsive at short distances [because the laws of quantum physics do not allow identical particles like electrons to occupy the same region of space]. A bound system of two atoms has E less than zero. At any point, E = K + U, so where E becomes equal to U, the classical kinetic energy must be zero. These points are therefore called “classical turning points.”
In physics we always define the potential energy between a pair of objects, due to a fundamental force acting between them, to be zero when the objects are an infinite distance apart. That is, U(r) → 0 as r → ∞. There is only one case (forces between quarks) where this is impossible!