 |
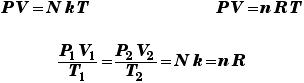
| |

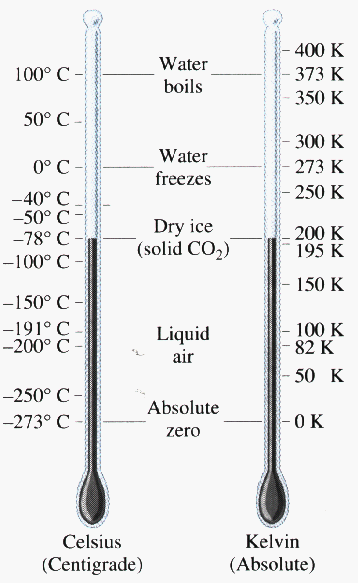
Trivial example: consider a gas with N = 1027 atoms, at a temperature of 300 K, occupying a volume of 1 m3. The gas pressure must then be p = (N kB T)/V which instantly works out to 4.1 x 106 N/m2 or 41 atmospheres.
What volume is occupied by 1 kilomole of a gas at standard temperature and pressure (1 atmosphere, 0 degrees C)? V = nRT/p where R is 8310 J/K and the temperature is 273 K. Then at once we get a volume of 22.5 m3 which is 22,500 liters. Thus a mole would indeed occupy 22,500/(1000) = 22.5 liters.